General aspects of Ion Selective Electrodes
The Ion Selective Electrodes are the potenciometric sensors that selective respond on the ion activity in the solution according to Nikolski-Eisenmann equation:
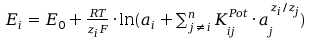 (1)
(1)
where Ei is the potencial of ion selective electrode, E0 is so called standart potential of the electrode, ai is the activity of the indicating ion, KijPot is the selectivity coefficient of the electrode toward j-th ion regarding to indicating ion, aj is the activity j-th interfering ion, zi is the charge of the indicating ion and zj is the charge j interfering ion, n in the sum means n types of interfering ions, R is universal gas constant, T is absolute temperature and F is Faraday constant. For the practical reason is advantegous use expression with decadic logarithm:
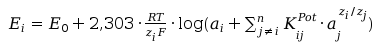 (2)
(2)
From the practical wiev is advantigous so that the contributions of the interfering ions were neglibile so that was be valid:
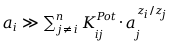 (3)
(3)
So then we can eqation rewrite:
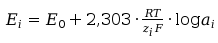 (4)
(4)
For the series measuring is advantige , when the activity coefficient is constant. Betwen ion activity and his concetration is the folowing relation:
a i = ci.γi. (5)
Then we can write:
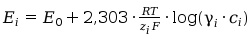 (6)
(6)
When we keep the activity coefficient constant, we can write:
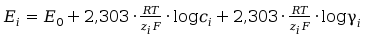 (7)
(7)
If the measuring pass in the same temperature the eqation can be written:
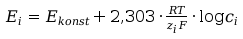 (8)
(8)
For this purpose are used some indiferent electrolytes which we add to solution. They are not interfere the determination, but they ensure constant ionic strenght during of the measuring. Some special mixtures of the electrolytes (TISAB,SAOB) not only fix ionic strenght, but as well mask for example ions, that can interfere determination.
First, known ion selective electrode was glass pH electrode on the base of the sodium calcium silicate glasses. When that pH was measured in alkaline region in sodium hydroxide, potassium hydroxide or lithium hydroxide the pH electrode shown so called alkaline error. This alkaline error is typical example of the interfering sodium, potassium and lithium ion on the function of the pH glass electrodes.The influence of these ions on the potential of the glass pH electrode for sodium ,potassium and lithium ions is diferent for the same electrode and depends on the value of the selectivity coefficients toward these ions regarding hydrogen ion for this electrode. When the composition of the sodium calcium silicate glasses is changed so that calcium ion is replaced by aluminium ion the electrode begin be sensitive to sodium ion. On this base are produced sodium ion selective electrodes.
Tel: +420 481 325 857, Tel/Fax: +420 481 313 200, Mobile: +420 775 325 857